Tuesday, August 22, 2006
My Pain is my prisoner
It makes me to lie down between hospital sheets
It destroyeth my soul:
It loses me in the paths of sickness, I’m sick of its name Even tho I walk down the hospital corridors is till fear evil,
Tho my drip is with me its pole and its bad don’t comfort me
They prepare others tables in the presence of my nausea
They assault my body with drugs, my patience runs out.
Surely illness and despair will leave me and I will dwell in this house of sickness no MORE!!
This was posted on the PMB with a request to find out who the orginal Author was. So if you can help then please feel free to post a comment. Thanks for your help.
Wednesday, August 02, 2006
What does S.O.D. Stand for?
In Reply to: ps. what does sod stand for? posted by jeff on July 24, 2006 at
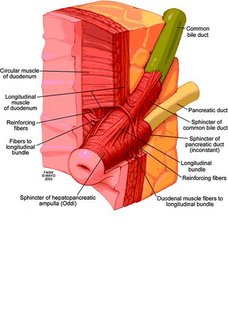
Sphincter of oddi disfunction.. its also on jerrys blog.. I'll put the pic here and then paste the info in this post**************************************************
This is a picture that shows the sphincter of oddi. the main pancreatic duct and bile duct from the liver both converge and their liquids are released via the sphincter. The main pancreatic duct will back up with activated enzymes and they will eat and destroy all along the length of the pancreas. In the torso diagram, where the head of the pancreas is (torsos right side) Jerry has a knot that protrudes out of his abdomen. We were todl that the pancreas is swelling so large that it pushes out from under the stomach and that is what is causing the bulge.*******************************************
ps the bulge is gone and has not returned! so it was the pancreas and NOT a food bolus!
Insurance Help Sites
Patient assistance programs-helpingpatients.org/
--------------------------------------------------------------------------------
www.togetherrxaccess.com/
www.medicare.gov
www.disabilityresources.org To find disability organizations or agenices in your area, click on your home state:
http://www.ncpad.org/organizations/alpha
http://www.focusas.com/HealthInfoNumbers.htm Toll-Free Numbers for Health Information
http://www.3m.com/us/healthcare/pha...ssistance.jhtml
http://sis.nlm.nih.gov/hotlines/
http://coveringkidsandfamilies.org/communications/bts/
http://www.freemedicineprogram.com/drug/ELDER+TONIC/
http://www.mycancernews.com/viatical.html
http://www.cancercare.org/HelpingHa...deList.cfm?c=49
http://www.helpingpatients.org/
http://www.rxassist.org/default.cfm
http://www.doctorhealthynet.com/fre...dicine_home.htm
http://www.needymeds.com/
http://www.mhsanctuary.com/add/rx.htm
http://www.astrazeneca-us.com/content/drugAssistance/
https://www.merck.com/pap/pap/consu...application.jsp
http://www.pfizer.com/subsites/phil...ents.index.html
http://www.sch-plough.com/schering_...nt_programs.jsp
http://www.phrma.org/
http://www.hrsa.gov/osp/dfcr/obtain/obtain.htm
http://www.lillyanswers.com/
http://www.benefitscheckup.com/
http://www.pharma.us.novartis.com/i...?TNav&checked=y
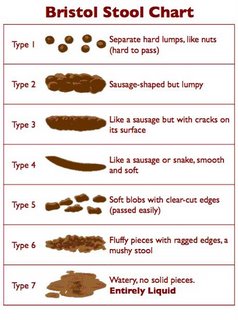
Bristol Stool Scale
Bristol Stool ScaleFrom Wikipedia, the free encyclopediaYou have new messages (last change).Jump to: navigation, search
The Bristol Stool Scale or Bristol Stool Chart is a medical aid designed to classify the faeces form into seven groups. It was developed by Heaton and Lewis at the University of Bristol and was first published in the journal Scand J Gastroenterol in 1997. Because the form of the stool depends on the time it spends in the colon, there is a correlation between the colonic transit time and the stool type.
The seven types of stool are:
Type 1: Separate hard lumps, like nuts (hard to pass)
Type 2: Sausage-shaped, but lumpy
Type 3: Like a sausage but with cracks on its surface
Type 4: Like a sausage or snake, smooth and soft
Type 5: Soft blobs with clear cut edges (passed easily)
Type 6: Fluffy pieces with ragged edges, a mushy stool
Type 7: Watery, no solid pieces (entirely liquid)
Types 1 and 2 indicate constipation, with 3 and 4 being the "ideal stools" especially the latter, as they are the easiest to pass. 5-7 being further tending towards diarrhoea.
[edit]ReferencesConstipation Management and Nurse Prescribing: The importance of developing a concordant approach PDF Faecal incontinence and constipation PDF [edit]External linksThe Bristol Stool Scale from Medscape.com Information from Solvay Pharmaceuticals Childhood Constipation Retrieved from "http://en.wikipedia.org/wiki/Bristol_Stool_Scale"Categories: General practice | Gastroenterology
Ambry Test
http://www.ambrygen.com/reports/Pancreatitis%20Sales%20Aid%205-06.pdf
The Ambry Test : PancreatitisPancreatitis is a serious problem with asignificant genetic component.Pancreatitis accounts for 87,000 hospitalizationsper year in the US.1 Chronic pancreatitis patientsface a 26-fold increased risk of pancreatic cancer,and for those with hereditary pancreatitis, the riskis 50-fold with approximately 40% developingpancreatic cancer by age 70.2Normal exocrine pancreatic function depends on adelicate balance of enzyme activation andinhibition which can be affected by multipleenvironmental and genetic factors. Hereditarypancreatitis has been linked to mutations in thePRSS1 gene. Study of idiopathic chronic andrecurrent acute pancreatitis demonstrates thatmutations in three genes – PRSS1, SPINK1, andCFTR – predispose to pancreatitis.3Due to the cumulative contribution of three genesin both dominant and recessive patterns, a patientmay have genetically-determined pancreatitis evenif family history is negative.Ambry Genetics offers the world’s onlycomprehensive genetic test for pancreatitis.To help determine etiology and suggestappropriate treatments, Ambry Genetics offersanalysis of all coding regions of the CFTR, PRSS1,and SPINK1 genes in The Ambry Test: PancreatitisAMPLIFIED™. This test also analyzes the CFTRgene for gross deletions and duplications, whenindicated, providing a detection rate ofapproximately 99% for each gene. The panel isalso available without CFTR deletion/duplicationanalysis (The Ambry Test: Pancreatitis, CFdetection rate 97-98%), and each test is availableseparately.100 Columbia #200 | Aliso Viejo, CA 92656 | 949 900 5500fax 949 900 5501 | toll free 866 262 7943 |
www.ambrygen.com|
Pancreatitis1 in 4 patients tests positive for at least onesignificant genetic mutation.Mutations in three genes predispose to chronicand recurrent acute pancreatitis:• CFTR – cystic fibrosis transmembraneconductance regulator• PRSS1 – cationic trypsinogen• SPINK1 – serine protease inbitor, Kazal type 1Genetic testing for pancreatitis can help you:• Avoid repeated diagnostic testing• Provide targeted therapy• Address increased cancer risk in geneticallydeterminedpancreatitis• Help your patient understand his condition andincrease compliance• Communicate inheritance risks tofamily membersNo other test can identifymore mutations thanThe Ambry Test:Pancreatitis AMPLIFIEDGet started with Ambry Genetics today.Sample submission kits are available atno charge. Please call 866-262-7943 or visitour website to obtain more information andall necessary forms.™100 Columbia #200 | Aliso Viejo, CA 92656 | 949 900 5500fax 949 900 5501 | toll free 866 262 7943 | www.ambrygen.com|
Pancreatitis• In the entire patient set, 49.1% (116/236) carriedat least one mutation.• 11.0% (26/236) had a form of CF confirmed by2 CF mutations. An additional 22.0% (52/236)had one CF mutation.• 8.9% (21/236) had mutations in more than one gene.• Only 4.2% (10/236) patients had PRSS1mutations only.• Approximately 1/4 patients (23.7%, 56/256) havemutations with a clear causative component(defined as presence of 2 CF mutations and/orknown deleterious PRSS1 or SPINK1 mutations).83891, 83894, 83898,83903, 83904, 83909,839123-5 WeeksFull gene mutation scanning is performed by modified TemporalTemperature Gradient Electrophoresis (mTTGE). All sequencevariations detected by mTTGE are identified by double-strandedautomated sequencing. If indicated, CFTR gross deletion /duplication testing is performed by MLPA (MRC Holland).Blood: 5cc peripheral blood in purple-top EDTA (preferred) oryellow top ACD. Store at 2-8° C up to 96 hours prior toshipping. Do not freeze. Ship at room temperature.CPT CodesTurn-Around-TimeMethodSpecimenRequirements1 Yakshe P. Pancreatitis, chronic. emedicine [online resource]. Available at: http://www.emedicine.com/MED/topic1721.htm. Last update July 2005.2 Erickson RA. Pancreatic cancer. emedicine [online resource]. Available at: http://www.emedicine.com/MED/topic1712.htm. Last update Dec 2005.3 Etemad B, Whitcomb DC. Gastroenterology. 2001;120:682-707.4 Whitcomb DC.US Gastro Review. 2006;56-58, and personal communication.© 2006 Ambry Genetics. All rights reserved. P0506-09-003-MKG-00.PANCREATITIS PANEL PANCREATITIS PANEL AMPLIFIED83891, 83894, 83898, 83900,83901, 83903, 83904, 83909,839124-6 Weeks1 in 4 patients has at least onesignificant mutation.In a series of patients with chronic or recurrentacute pancreatitis, The Ambry Test: Pancreatitisdetected at least one mutation in 49.1% (116/236).Though patients known to have cystic fibrosis (CF)were excluded from this series, 11.0% (26/236) werefound to have a form of CF confirmed with at leasttwo CFTR mutations. Nearly as many patients(8.9%, 21/236) had mutations in more thanone gene.Using a stricter definition of clinical significance aspresence of known deleterious mutations in PRSS1or SPINK1, and/or two CF mutations, approximatelyone in four patients (23.7%, 56/236) has a causativegenetic component to their pancreatitis.Genetic testing results can help youmanage your patient.As mutations in different genes affect differentsteps of trypsin activation and inhibition, genetictesting can suggest treatments targeted to apatient’s particular defect. For example, PRSS1mutations may lead to premature trypsinogenactivation through altered sensitivity to calcium.Preventive measures could include various steps tominimize stimulation of the acinar cells and assistcalcium regulation.4 CFTR mutations impairpancreatic duct flushing, so treatments could focuson stimulating the pancreas and maximizing flowthrough the duct.4Identifying patients with mutations allowsappropriately increased cancer surveillance.Knowledge of contributory mutations explains thedisease to the patient, reinforces understanding ofits chronic nature, and affirms the importance ofcompliance with dietary and lifestyle modifications.Further, family members may be tested andcounseled to minimize their risk of developingchronic pancreatitis.Mutation distribution in 236 patients testedwith The Ambry Test: Pancreatitits
The Ambry Test : PancreatitisPancreatitis is a serious problem with asignificant genetic component.Pancreatitis accounts for 87,000 hospitalizationsper year in the US.1 Chronic pancreatitis patientsface a 26-fold increased risk of pancreatic cancer,and for those with hereditary pancreatitis, the riskis 50-fold with approximately 40% developingpancreatic cancer by age 70.2Normal exocrine pancreatic function depends on adelicate balance of enzyme activation andinhibition which can be affected by multipleenvironmental and genetic factors. Hereditarypancreatitis has been linked to mutations in thePRSS1 gene. Study of idiopathic chronic andrecurrent acute pancreatitis demonstrates thatmutations in three genes – PRSS1, SPINK1, andCFTR – predispose to pancreatitis.3Due to the cumulative contribution of three genesin both dominant and recessive patterns, a patientmay have genetically-determined pancreatitis evenif family history is negative.Ambry Genetics offers the world’s onlycomprehensive genetic test for pancreatitis.To help determine etiology and suggestappropriate treatments, Ambry Genetics offersanalysis of all coding regions of the CFTR, PRSS1,and SPINK1 genes in The Ambry Test: PancreatitisAMPLIFIED™. This test also analyzes the CFTRgene for gross deletions and duplications, whenindicated, providing a detection rate ofapproximately 99% for each gene. The panel isalso available without CFTR deletion/duplicationanalysis (The Ambry Test: Pancreatitis, CFdetection rate 97-98%), and each test is availableseparately.100 Columbia #200 | Aliso Viejo, CA 92656 | 949 900 5500fax 949 900 5501 | toll free 866 262 7943 | www.ambrygen.com| Pancreatitis
1 in 4 patients tests positive for at least onesignificant genetic mutation.Mutations in three genes predispose to chronicand recurrent acute pancreatitis:• CFTR – cystic fibrosis transmembraneconductance regulator• PRSS1 – cationic trypsinogen• SPINK1 – serine protease inbitor, Kazal type 1Genetic testing for pancreatitis can help you:• Avoid repeated diagnostic testing• Provide targeted therapy• Address increased cancer risk in geneticallydeterminedpancreatitis• Help your patient understand his condition andincrease compliance• Communicate inheritance risks tofamily membersNo other test can identifymore mutations thanThe Ambry Test:Pancreatitis AMPLIFIEDGet started with Ambry Genetics today.Sample submission kits are available atno charge. Please call 866-262-7943 or visitour website to obtain more information andall necessary forms.™100 Columbia #200 | Aliso Viejo, CA 92656 | 949 900 5500fax 949 900 5501 | toll free 866 262 7943 | www.ambrygen.com| Pancreatitis•
In the entire patient set, 49.1% (116/236) carriedat least one mutation.• 11.0% (26/236) had a form of CF confirmed by2 CF mutations. An additional 22.0% (52/236)had one CF mutation.• 8.9% (21/236) had mutations in more than one gene.• Only 4.2% (10/236) patients had PRSS1mutations only.• Approximately 1/4 patients (23.7%, 56/256) havemutations with a clear causative component(defined as presence of 2 CF mutations and/orknown deleterious PRSS1 or SPINK1 mutations).83891, 83894, 83898,83903, 83904, 83909,839123-5 WeeksFull gene mutation scanning is performed by modified TemporalTemperature Gradient Electrophoresis (mTTGE). All sequencevariations detected by mTTGE are identified by double-strandedautomated sequencing. If indicated, CFTR gross deletion /duplication testing is performed by MLPA (MRC Holland).Blood: 5cc peripheral blood in purple-top EDTA (preferred) oryellow top ACD. Store at 2-8° C up to 96 hours prior toshipping. Do not freeze. Ship at room temperature.CPT CodesTurn-Around-TimeMethodSpecimenRequirements1 Yakshe P. Pancreatitis, chronic. emedicine [online resource]. Available at: http://www.emedicine.com/MED/topic1721.htm. Last update July 2005.2 Erickson RA. Pancreatic cancer. emedicine [online resource]. Available at: http://www.emedicine.com/MED/topic1712.htm. Last update Dec 2005.3 Etemad B, Whitcomb DC. Gastroenterology. 2001;120:682-707.4 Whitcomb DC.US Gastro Review. 2006;56-58, and personal communication.© 2006 Ambry Genetics. All rights reserved. P0506-09-003-MKG-00.PANCREATITIS PANEL PANCREATITIS PANEL AMPLIFIED83891, 83894, 83898, 83900,83901, 83903, 83904, 83909,839124-6 Weeks
1 in 4 patients has at least onesignificant mutation.In a series of patients with chronic or recurrentacute pancreatitis, The Ambry Test: Pancreatitisdetected at least one mutation in 49.1% (116/236).Though patients known to have cystic fibrosis (CF)were excluded from this series, 11.0% (26/236) werefound to have a form of CF confirmed with at leasttwo CFTR mutations. Nearly as many patients(8.9%, 21/236) had mutations in more thanone gene.Using a stricter definition of clinical significance aspresence of known deleterious mutations in PRSS1or SPINK1, and/or two CF mutations, approximatelyone in four patients (23.7%, 56/236) has a causativegenetic component to their pancreatitis.Genetic testing results can help youmanage your patient.As mutations in different genes affect differentsteps of trypsin activation and inhibition, genetictesting can suggest treatments targeted to apatient’s particular defect. For example, PRSS1mutations may lead to premature trypsinogenactivation through altered sensitivity to calcium.Preventive measures could include various steps tominimize stimulation of the acinar cells and assistcalcium regulation.4 CFTR mutations impairpancreatic duct flushing, so treatments could focuson stimulating the pancreas and maximizing flowthrough the duct.4Identifying patients with mutations allowsappropriately increased cancer surveillance.Knowledge of contributory mutations explains thedisease to the patient, reinforces understanding ofits chronic nature, and affirms the importance ofcompliance with dietary and lifestyle modifications.Further, family members may be tested andcounseled to minimize their risk of developingchronic pancreatitis.Mutation distribution in 236 patients testedwith The Ambry Test: Pancreatitits
Celiac Plexus Block
In Reply to: Celiac Plexus Nerve Blocks posted by Robin H. on August 01, 2006 at 10:42:48:
(image placeholder)
I knew someone on here had had something diff done. Thats why i told jeff, someone else will pipe up with info. also i found this neat page!!
*****************************************************ROUND TABLE
Article in PDF format - JOP Home page
JOP. J Pancreas (Online) 2004; 5(4):315-321.
Celiac Plexus Neurolysis
Paolo Giorgio Arcidiacono, Marzia Rossi
Diagnostic and Therapeutic Endosonography Unit, Division of Gastroenterology and Gastrointestinal Endoscopy, Vita-Salute San Raffaele University - San Raffaele Hospital. Milan, Italy
Introduction
Pancreatic cancer is the tenth most common malignancy and the fourth cause of cancer-related death in Western countries. Because 5-year survival in referral centers is less than 30%, clinical management of most patients involves palliation of the symptoms of which 90% are weight loss, jaundice, and pain.
While jaundice related to biliary obstruction can be palliated by means of endoscopic therapy or surgery, pancreatic pain is often difficult to control.
Initial therapy with non-steroid anti-inflammatory agents (NSAIDs) is often rapidly overwhelmed by pain and necessitates being associated with opioid administration.
Although opioids effectively relieve pain, they are associated with many different collateral effects, such as dry mouth, constipation, nausea, vomiting, drowsiness and delirium, which can determine a great decrease in quality of life and may also impair the immune function. Pancreatic pain is also quite common in patients with chronic pancreatitis and, in this case, pain has a multi-factorial etiology; for this reason, prolonged drug therapy is related to an increased risk of narcotic-dependence [1].
Celiac plexus neurolysis (CPN) is a chemical splanchnicectomy of the celiac plexus; its goal is to ablate the efferent nerve fibres which transmit pain from the intra-abdominal viscera.
Although the terms "celiac plexus" and "splanchnic nerves" are often used interchangeably, these are anatomically distinct structures.
The splanchnic nerves are located above the diaphragm (retro-crural) and are typically anterior to the 12th thoracic vertebra; on the other hand, the celiac plexus is situated below the diaphragm (ante-crural), surrounding the basis of the celiac trunk. This plexus is composed of a dense network of ganglia and interconnecting fibres (Figure 1).
Figure 1. The celiac plexus.
The ganglia vary in number (1-5), size (diameter 0.5-4.5 cm), and location (T12-L2), but, independently on their size, the ganglia cannot be visualized as distinct structures by any kind of imaging modality.
The celiac plexus transmits pain sensation originating from the pancreas and most of the abdominal viscera except for the left colon, rectum and pelvic organs.
Stimuli reach the thalamus and the cortex of the brain, leading to pain sensation. On the contrary, some descending inhibitory mechanisms may also modulate the ascending pain information.
The CPN technique was first described by Kappis et al. in 1919 [2]; since then, a number of modifications have been proposed and introduced in a clinical setting in an attempt to improve the accuracy of needle placement and pain relief while reducing procedure-related complications.
Nowadays, CPN is most commonly used to palliate patients suffering from pain due to pancreatic cancer and chronic pancreatitis; it can be performed using different approaches either percutaneously, surgically or under EUS guidance. Until the 1990s, the most common of the above was surely the percutaneous route, injecting absolute alcohol into the celiac plexus under fluoroscopy or CT guidance.
Different studies have reported data on safety, accuracy in reaching the right site of injection and efficacy in decreasing pain due to different diseases by means of CT-guided CPN.
Some authors described 28 cases of CPN performed under CT guidance in patients having neoplasms originating in the pancreas (n=10), stomach (n=8), bile ducts (n=5), liver (n=3), right colon (n=1) and kidney (n=1) [3]. The study showed that this procedure is safe and efficient in controlling pain [3].
Unfortunately, the CT CPN approach is usually posterior and, for this reason, cases of paraplegia have been reported caused by the puncture of the nervous radix at the time of the introduction of the needle during the procedure [4].
Endoscopic ultrasonography (EUS) is a relatively new imaging technique which couples a high frequency ultrasound probe with an oblique viewing endoscopic instrument. This combination allows the endoscopist to obtain a perfect evaluation of the pancreatic parenchyma and surrounding structures, not least, the aorta and celiac trunk. This imaging modality has achieved wide acceptance as the technique of choice for the evaluation of pancreatic disease, diagnosis and staging of pancreatic cancer, diagnosis of idiopathic pancreatitis and the evidencing of neuro-endocrine neoplasms.
At the beginning of EUS, instruments were provided by radial scanning probes; this means that the scanning plane of these probes was transversal, that is, perpendicular to the longitudinal axis of the endoscopic instrument.
This probe orientation absolutely limited the possibility of these instruments performing EUS guided diagnostic or therapeutic procedures, due to the inability of the probe to follow, under real time guidance, the route of a needle device from the orifice of the working channel of the EUS instrument to a target lesion located either inside the gut wall or, as for CPN, outside the gut wall. In early 1990s, there was a technical revolution in EUS instrumentation; in fact, a longitudinal echoendoscope was presented.
This instrument was made with an electronic convex high frequency probe having a longitudinal scanning plane; this means that the scanning plane was on the same longitudinal axis as the endoscope and, more importantly, on the same axis of the working channel.
This innovation has opened the field of operative EUS, allowing the possibility of following, under real time guidance, any kind of device passed throughout the working channel to reach a target lesion.
Since that time, EUS has been tested in this new operative setting for many reasons, mainly the cytological analysis of tumors and, more recently, it has been applied in the treatment of pain in patients with chronic pancreatitis or pancreatic cancer by injecting neurolytic agents in the area of the celiac plexus.
Although many studies demonstrate that celiac plexus neurolysis effectively controls pancreatic cancer pain, up to 1% of patients undergoing percutaneous CPN may develop serious complications, including lower extremity weakness, paresthesias, including epidural anesthesia, lumbar puncture and pneumothorax.
In theory, EUS CPN is safer than posterior percutaneous techniques because EUS allows direct access to the celiac plexus without risk to the vital spinal nerves, the diaphragm or the spinal arteries.
Procedure
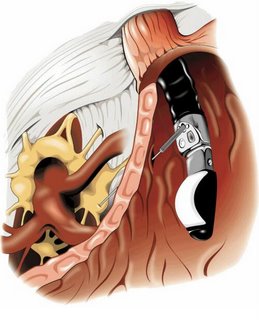
EUS-guided celiac plexus neurolysis (EUS CPN) is usually combined with the biopsy of a pancreatic primary lesion for diagnostic and staging purposes. It is performed with a linear array echoendoscope (Figure 2).
Figure 2. Linear array echoendoscope.
With these instruments, it is possible to follow, under EUS real time guidance, the route of the needle through the pancreatic lesion.
Informed consent is obtained with specific attention to complications associated with CPN and EUS guided fine needle aspiration (FNA) of pancreatic lesions. The procedure is performed under deep sedation under the supervision of an anesthesiologist. The patient lays on left lateral decubitus and his/her vital parameters are monitored.
Under direct endoscopic view, the linear EUS instrument is introduced into the stomach to reach the lesser curve in the sub-cardiac area. In this position, the probe is lightly pressed against the gastric wall to obtain a good coupling and a good view of surrounding structures. At this site, it is easy to identify the aorta under the diaphragm which appears as an anechoic tube structure in a longitudinal plane and the origin of the celiac axis is seen beside this. Color Doppler can confirm the vascular landmarks (Figures 3 and 4).
Figure 3. Monochrome visualization of the celiac trunk.
Figure 4. Color Doppler visualization of the celiac trunk.
As previously emphasized, the celiac plexus is not identified as a discrete structure but is located based on its position relative to the celiac trunk.
Two different treatment procedures have been described to perform EUS CPN depending on the device used to perform alcohol injection.
The first technique described uses a standard 22 gauge needle used for all the biopsy procedures under EUS guidance (Figure 5); this is a cutting needle with a removable inner sheet occluding a single hole at the needle tip. For this reason, it is necessary to perform two injections of alcohol at both sides of the trunk in order to obtain an adequate injection of alcohol at both sides of the celiac trunk.
Figure 5. Standard 22 gauge needles.
The second procedure, which is actually more diffused due to its rapidity, uses a new needle (Figure 6) properly designed for this procedure (EUS 20 CPN, Wilson Cook, Winston-Salem, NC, USA); it is a 20 gauge needle with a penetrating tip closed and with some lateral holes which allow a radial diffusion of alcohol to both sides of the origin of the celiac axis with a single injection.
Figure 6. EUS 20 CPN (Wilson Cook, Winston-Salem, NC, USA) needle.
Once the origin of the celiac trunk is located from the sub-cardiac position, the needle (whichever used) is passed through and fixed to the celiac trunk by a luer-lock. Then, under real time control, the needle is released and pushed out from the working channel to trans-pass the gastric posterior wall and is immediately inserted adjacent to the celiac trunk.
At this phase, the two procedures differ slightly, considering the needle position with respect to the celiac trunk; in the first procedure, due to the fact that the needle has only one hole and cannot spray alcohol, the needle tip is positioned by one side of the trunk originating from the aorta and, after having completed the injection, it must be pulled back slightly and again inserted on the other side of the trunk to carry out another injection.
In the second technique, the spraying possibility given by the EUS 20 CPN needle allows the endosonographer to put the needle tip anterior to the basis of the origin of the celiac axis and to carry out only one injection (Figure 7).
Figure 7. EUS image of the needle at base of the celiac trunk.
The injection time is identical for both devices used. When the needle tip is in place, the inner sheet is removed and an aspiration test is performed to rule out vessel penetration before injection.
Then, 3-6 mL of a local analgesic, usually bupivacaine 0.25-0.75%, is injected first followed by 15-20 mL of a neurolytic agent (98%) dehydrate alcohol (Figure 8).
Figure 8. EUS image of lidocaine injection.
The alcohol injection produces an echogenic cloud obscuring the aorta and celiac axis (Figure 9).
Figure 9. EUS image of alcohol injection showing an echogenic cloud obscuring aorta and celiac axis.
In chronic pancreatitis patients, some Authors prefer to use steroids (10 mL or 80 mg (6 mL) triamcinolone) instead of alcohol.
However, in chronic pancreatitis, most results are obtained with alcohol and when traditional techniques were used.
The EUS CPN procedure usually lasts approximately 15 minutes and, during the procedure, arterial pressure has to be monitored because the alcohol injection may produce hypotension and it is necessary to infuse saline solution.
Results
Currently, there are few data about CPN under EUS guidance. However, the results are comparable to other conventional methods used to relieve pancreatic pain with neurolytic agent injections.
The safety and efficacy of EUS CPN has previously been demonstrated as relieving pancreatic pain in a cohort of 25 patients with pancreatic cancer followed for 12 weeks and 5 patients with other intra-abdominal malignant neoplasms [5]. These studies showed a significant decrease of pain at 2, 4, 8 and 12 weeks after EUS CPN.
About 80% of the patients also benefited in a long-term observation (a mean follow up-of 10 weeks) [5]. Other Authors described 58 patients treated in order to palliate pain due to non-operable pancreatic cancer. A short-term decrease of pain was seen in 78% of the patients but the control of pain decreased in 30% at 12 weeks.
Of particular interest is the evidence, reported by Gunaratnam et al. in the widest population reported to have been treated using this modality, that, if the treatment is associated with chemoradiation or chemotherapy, the decrease in the pain score was significantly higher as compared to patients who did not undergo any additional therapy [6] (Figure 10).
Figure 10. Decrease of the pain score according to different treatments. (N Gunaratnam et al. [6], modified).
Up to now, the real role of EUS CPN in the treatment of pain related to chronic pancreatitis is not so clear, lacking enough comparative data comparing EUS CPN and other modalities of treating such multifactorial pain.
Only two studies [7, 8] have tested EUS CPN in this setting; in 90 patients steroids were injected during EUS CPN and a beneficial effect was observed after 7 days in only 55% [7]. Furthermore, in the follow-up of these patients, only 25% still showed a significant decrease in the pain score after 12 weeks [7].
Many authors believe that the difference in results between patients with pancreatic cancer or chronic pancreatitis probably depends on the origin of the pain which can be considered only due to the nervous growing of the tumor in pancreatic cancer and which is multifactorial, with a great psychological impact, in chronic pancreatitis.
Another study described 22 cases of CPN (10 EUS CPN and 12 CT CPN) which showed the benefit in 40% at 8 weeks under EUS guidance (30% at 24 weeks) and in 25% under CT guidance. But the Authors concluded that the number of patients was too limited [8].
There are only a few complications related to the procedure and they are described as only transitory [3]. Orthostatic hypotension or a transient diarrhea may frequently be described.
The infusion of liquid can contrast the hypotension, while the diarrhea is generally auto-limiting and does not exceed 24 hours. Only a few cases of chronic diarrhea have been described; other complications are: peri-pancreatic abscess, reversible paraparesis and a pseudo alcohol-induced aneurysm [9, 10, 11]. To prevent the abscess, it is important to carry out antibiotic prophylaxis.
Conclusions
Celiac plexus neurolysis during EUS appears to be a safe technique, without complications. It seems to control neoplastic pancreatic pain in a short time in about 90% of cases and in a long time in about 30%.
In the management of chronic pancreatitis pain, the role of EUS CPN is not so clear and only 50% of patients have a good reduction of pain within a short period of time. However, only 10% seem to show a benefit at 24 weeks. EUS CPN results seem to be comparable to the results obtained with the other procedures, although the numbers are still too low. An important advantage is that EUS CPN may be performed during bioptic staging of pancreatic cancer [12].
It is also not very clear if there is the possibility of performing more than one procedure of neurolysis.
In summary, EUS CPN is safe and effective for the palliation of patients with pain caused by unresectable pancreatic cancer. Chemotherapy with and without radiotherapy also significantly decreased pain scores.
In addition, the proximity of the posterior lesser curve of the stomach to the celiac plexus, the use of continuous real time visualization of the target area and the availability of the Doppler to assess the vasculature all facilitate accurate needle placement.
CPN under EUS guidance requires further investigation in order to identify the advantages of this approach over conventional percutaneous techniques. The ability to perform the procedure in conjunction with tumor staging and FNA may streamline the care of these patients. The use of EUS CPN earlier in the course of pancreatic cancer to alleviate pain should be encouraged.
References
Levy MJ, Wiersema MJ. EUS-guided celiac plexus neurolysis and celiac plexus block. Gastrointest Endosc 2003; 57:923-9. [More details]
Kappis M. Sensibilitat und lokale Anasthesie im chirurgischen Gebiet der Bauchhohle mit besonderer Berucksichtigung der splanchnicus-Aasthesie. Beitrage zur klinischen Chirurgie 1919; 115:161-75. [More details]
Lee JM. CT-guided celiac plexus block for intractable abdominal pain. J Korean Med Sci 2000; 15:173-8. [More details]
Eisenberg E, Carr DB, Chalmers CT. Neurolytic celiac plexus block for treatment of cancer pain: a meta-analysis. Anesth Analg 1995; 80:290-5. [More details]
Wiersema MJ, Wiersema LM. Endosonography-guided celiac plexus neurolysis. Gastrointest Endosc1996; 44:656-62. [More details]
Gunaratnam NT, Sarma AV, Norton ID, Wiersema MJ. A prospective study of EUS-guided celiac plexus neurolysis for pancreatic cancer pain. Gastrointest Endosc 2001; 54:316-24. [More details]
Gress F, Schmitt C, Sherman S, Ciaccia D, Ikenberry S, Lehman G. Endoscopic ultrasound-guided celiac plexus block for managing abdominal pain associated with chronic pancreatitis: a prospective single center experience. Am J Gastroenterol 2001; 96:409-16. [More details]
Gress F, Schmitt C, Sherman S, Ikenberry S, Lehman G. A prospective randomized comparison of endoscopic ultrasound- and computed tomography-guided celiac plexus block for managing chronic pancreatitis pain. Am J Gastroentero. 1999; 94:900-5. [More details]
Chan VW. Chronic diarrhea: an uncommon side effect of celiac plexus block. Anesth Analg 1996; 82:205-7. [More details]
Navarro-Martinez J, Montes A, Comps O, Sitges-Serra A. Retroperitoneal abscess after neurolytic celiac plexus block from the anterior approach. Reg Anesth Pain Med 2003; 28:528-30. [More details]
Kumar A, Tripathi SS, Dhar D, Bhattacharya A. A case of reversible paraparesis following celiac plexus block. Reg Anesth Pain Med 2001; 26:75-8. [More details]
Varadarajulu S, Wallace MB. Applications of endoscopic ultrasonography in pancreatic cancer. Cancer Control 2004; 11:15-22. [More details]
Article in PDF format
--------------------------------------------------------------------------------
Keywords Celiac Plexus /innervation; Endosonography; Pain; Pancreatic Neoplasms
Abbreviations CPN: celiac plexus neurolysis
CorrespondencePaolo Giorgio ArcidiaconoDivision of Gastroenterology and Gastrointestinal EndoscopyUniversity Vita-Salute San RaffaeleIRCCS San Raffaele HospitalVia Olgettina, 6020132 MilanoItalyPhone: +39-02.2643.2145Fax: +39-02.215.2559E-mail address: arcidiacono.paologiorgio@hsr.it
JOP Home page
- http://www.joplink.net/prev/200407/26.html
Blood Work Questions
Posted by cj on
In Reply to: blood work question?? posted by jeff
Lab Studies:
Blood tests
**Elevations of serum amylase and lipase are found only during acute attacks of pancreatitis, usually early in the course of the disease**In the later stages of chronic pancreatitis, atrophy of the pancreatic parenchyma can result in serum
enzyme levels within the reference range, even during acute attacks of pain.
While low concentrations of serum trypsin are relatively specific for advanced chronic pancreatitis, they are not sensitive enough to be helpful in most patients with mild-to-moderate disease.
Laboratory studies to identify causative factors include serum calcium and triglyceride levels.
When common etiologies are not found, research protocols are available to test for genetic mutations in cationic trypsinogen and CFTR.
Fecal tests
Because maldigestion and malabsorption do not occur until more than 90% of the pancreas has been destroyed, steatorrhea is a manifestation of advanced chronic pancreatitis, and neither qualitative nor quantitative fecal fat analysis can detect early disease.
Assays of fecal chymotrypsin and human pancreatic elastase 1 have the same limitations but are useful in confirming advanced chronic pancreatitis with exocrine insufficiency.
Pancreatic function tests
Direct tests: These tests are the most sensitive and can be used to detect chronic pancreatitis at its earliest stage; however, they are somewhat invasive, labor intensive, and expensive.
Determination in duodenal aspirates: Intubation of the duodenum usually is performed with a Dreiling tube, which allows for separate aspiration of gastric and duodenal contents. The methodology varies depending on the specific laboratory; however, the authors generally use exogenous secretin with cerulein or cholecystokinin to achieve maximal stimulation of the pancreas. The output of pancreatic bicarbonate, protease, amylase, and lipase then is measured in the duodenal aspirates. This test currently only is available in specialized centers. While the greatest sensitivity can be obtained in prolonged infusions of secretagogue to uncover a decreased pancreatic secretory reserve, it is impractical for general clinical use.
Determination in pancreatic juice: This test generally is performed in conjunction with an endoscopic retrograde cholangiopancreatography (ERCP). The pancreatic duct is freely cannulated, an exogenous secretagogue is administered as above, and the pancreatic juice then is aspirated out of the duct as it is produced. The output of pancreatic bicarbonate, protease, amylase, and lipase are measured. This test is gaining popularity because most patients undergo ERCP at some point in their evaluation.
Indirect tests: Noninvasive tests of pancreatic function have been developed for detecting chronic pancreatitis. In principle, these tests work via oral administration of a complex substance that is hydrolyzed by a specific pancreatic enzyme to release a marker substance. The intestine absorbs the marker, which then is measured in the serum or urine. These tests are capable of detecting moderate-to-severe chronic pancreatitis. The presence of renal, intestinal, and liver disease may interfere with the accuracy of these tests. Neither currently is freely available in the
http://www.emedicine.com/med/topic1721.htm