In Reply to: Celiac Plexus Nerve Blocks posted by Robin H. on August 01, 2006 at 10:42:48:
(image placeholder)
I knew someone on here had had something diff done. Thats why i told jeff, someone else will pipe up with info. also i found this neat page!!
*****************************************************ROUND TABLE
Article in PDF format - JOP Home page
JOP. J Pancreas (Online) 2004; 5(4):315-321.
Celiac Plexus Neurolysis
Paolo Giorgio Arcidiacono, Marzia Rossi
Diagnostic and Therapeutic Endosonography Unit, Division of Gastroenterology and Gastrointestinal Endoscopy, Vita-Salute San Raffaele University - San Raffaele Hospital. Milan, Italy
Introduction
Pancreatic cancer is the tenth most common malignancy and the fourth cause of cancer-related death in Western countries. Because 5-year survival in referral centers is less than 30%, clinical management of most patients involves palliation of the symptoms of which 90% are weight loss, jaundice, and pain.
While jaundice related to biliary obstruction can be palliated by means of endoscopic therapy or surgery, pancreatic pain is often difficult to control.
Initial therapy with non-steroid anti-inflammatory agents (NSAIDs) is often rapidly overwhelmed by pain and necessitates being associated with opioid administration.
Although opioids effectively relieve pain, they are associated with many different collateral effects, such as dry mouth, constipation, nausea, vomiting, drowsiness and delirium, which can determine a great decrease in quality of life and may also impair the immune function. Pancreatic pain is also quite common in patients with chronic pancreatitis and, in this case, pain has a multi-factorial etiology; for this reason, prolonged drug therapy is related to an increased risk of narcotic-dependence [1].
Celiac plexus neurolysis (CPN) is a chemical splanchnicectomy of the celiac plexus; its goal is to ablate the efferent nerve fibres which transmit pain from the intra-abdominal viscera.
Although the terms "celiac plexus" and "splanchnic nerves" are often used interchangeably, these are anatomically distinct structures.
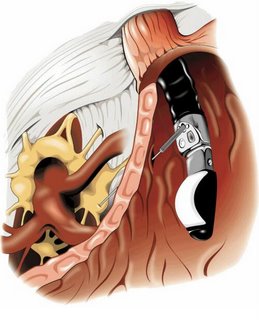
The splanchnic nerves are located above the diaphragm (retro-crural) and are typically anterior to the 12th thoracic vertebra; on the other hand, the celiac plexus is situated below the diaphragm (ante-crural), surrounding the basis of the celiac trunk. This plexus is composed of a dense network of ganglia and interconnecting fibres (Figure 1).
Figure 1. The celiac plexus.
The ganglia vary in number (1-5), size (diameter 0.5-4.5 cm), and location (T12-L2), but, independently on their size, the ganglia cannot be visualized as distinct structures by any kind of imaging modality.
The celiac plexus transmits pain sensation originating from the pancreas and most of the abdominal viscera except for the left colon, rectum and pelvic organs.
Stimuli reach the thalamus and the cortex of the brain, leading to pain sensation. On the contrary, some descending inhibitory mechanisms may also modulate the ascending pain information.
The CPN technique was first described by Kappis et al. in 1919 [2]; since then, a number of modifications have been proposed and introduced in a clinical setting in an attempt to improve the accuracy of needle placement and pain relief while reducing procedure-related complications.
Nowadays, CPN is most commonly used to palliate patients suffering from pain due to pancreatic cancer and chronic pancreatitis; it can be performed using different approaches either percutaneously, surgically or under EUS guidance. Until the 1990s, the most common of the above was surely the percutaneous route, injecting absolute alcohol into the celiac plexus under fluoroscopy or CT guidance.
Different studies have reported data on safety, accuracy in reaching the right site of injection and efficacy in decreasing pain due to different diseases by means of CT-guided CPN.
Some authors described 28 cases of CPN performed under CT guidance in patients having neoplasms originating in the pancreas (n=10), stomach (n=8), bile ducts (n=5), liver (n=3), right colon (n=1) and kidney (n=1) [3]. The study showed that this procedure is safe and efficient in controlling pain [3].
Unfortunately, the CT CPN approach is usually posterior and, for this reason, cases of paraplegia have been reported caused by the puncture of the nervous radix at the time of the introduction of the needle during the procedure [4].
Endoscopic ultrasonography (EUS) is a relatively new imaging technique which couples a high frequency ultrasound probe with an oblique viewing endoscopic instrument. This combination allows the endoscopist to obtain a perfect evaluation of the pancreatic parenchyma and surrounding structures, not least, the aorta and celiac trunk. This imaging modality has achieved wide acceptance as the technique of choice for the evaluation of pancreatic disease, diagnosis and staging of pancreatic cancer, diagnosis of idiopathic pancreatitis and the evidencing of neuro-endocrine neoplasms.
At the beginning of EUS, instruments were provided by radial scanning probes; this means that the scanning plane of these probes was transversal, that is, perpendicular to the longitudinal axis of the endoscopic instrument.
This probe orientation absolutely limited the possibility of these instruments performing EUS guided diagnostic or therapeutic procedures, due to the inability of the probe to follow, under real time guidance, the route of a needle device from the orifice of the working channel of the EUS instrument to a target lesion located either inside the gut wall or, as for CPN, outside the gut wall. In early 1990s, there was a technical revolution in EUS instrumentation; in fact, a longitudinal echoendoscope was presented.
This instrument was made with an electronic convex high frequency probe having a longitudinal scanning plane; this means that the scanning plane was on the same longitudinal axis as the endoscope and, more importantly, on the same axis of the working channel.
This innovation has opened the field of operative EUS, allowing the possibility of following, under real time guidance, any kind of device passed throughout the working channel to reach a target lesion.
Since that time, EUS has been tested in this new operative setting for many reasons, mainly the cytological analysis of tumors and, more recently, it has been applied in the treatment of pain in patients with chronic pancreatitis or pancreatic cancer by injecting neurolytic agents in the area of the celiac plexus.
Although many studies demonstrate that celiac plexus neurolysis effectively controls pancreatic cancer pain, up to 1% of patients undergoing percutaneous CPN may develop serious complications, including lower extremity weakness, paresthesias, including epidural anesthesia, lumbar puncture and pneumothorax.
In theory, EUS CPN is safer than posterior percutaneous techniques because EUS allows direct access to the celiac plexus without risk to the vital spinal nerves, the diaphragm or the spinal arteries.
Procedure
EUS-guided celiac plexus neurolysis (EUS CPN) is usually combined with the biopsy of a pancreatic primary lesion for diagnostic and staging purposes. It is performed with a linear array echoendoscope (Figure 2).
Figure 2. Linear array echoendoscope.
With these instruments, it is possible to follow, under EUS real time guidance, the route of the needle through the pancreatic lesion.
Informed consent is obtained with specific attention to complications associated with CPN and EUS guided fine needle aspiration (FNA) of pancreatic lesions. The procedure is performed under deep sedation under the supervision of an anesthesiologist. The patient lays on left lateral decubitus and his/her vital parameters are monitored.
Under direct endoscopic view, the linear EUS instrument is introduced into the stomach to reach the lesser curve in the sub-cardiac area. In this position, the probe is lightly pressed against the gastric wall to obtain a good coupling and a good view of surrounding structures. At this site, it is easy to identify the aorta under the diaphragm which appears as an anechoic tube structure in a longitudinal plane and the origin of the celiac axis is seen beside this. Color Doppler can confirm the vascular landmarks (Figures 3 and 4).
Figure 3. Monochrome visualization of the celiac trunk.
Figure 4. Color Doppler visualization of the celiac trunk.
As previously emphasized, the celiac plexus is not identified as a discrete structure but is located based on its position relative to the celiac trunk.
Two different treatment procedures have been described to perform EUS CPN depending on the device used to perform alcohol injection.
The first technique described uses a standard 22 gauge needle used for all the biopsy procedures under EUS guidance (Figure 5); this is a cutting needle with a removable inner sheet occluding a single hole at the needle tip. For this reason, it is necessary to perform two injections of alcohol at both sides of the trunk in order to obtain an adequate injection of alcohol at both sides of the celiac trunk.
Figure 5. Standard 22 gauge needles.
The second procedure, which is actually more diffused due to its rapidity, uses a new needle (Figure 6) properly designed for this procedure (EUS 20 CPN, Wilson Cook, Winston-Salem, NC, USA); it is a 20 gauge needle with a penetrating tip closed and with some lateral holes which allow a radial diffusion of alcohol to both sides of the origin of the celiac axis with a single injection.
Figure 6. EUS 20 CPN (Wilson Cook, Winston-Salem, NC, USA) needle.
Once the origin of the celiac trunk is located from the sub-cardiac position, the needle (whichever used) is passed through and fixed to the celiac trunk by a luer-lock. Then, under real time control, the needle is released and pushed out from the working channel to trans-pass the gastric posterior wall and is immediately inserted adjacent to the celiac trunk.
At this phase, the two procedures differ slightly, considering the needle position with respect to the celiac trunk; in the first procedure, due to the fact that the needle has only one hole and cannot spray alcohol, the needle tip is positioned by one side of the trunk originating from the aorta and, after having completed the injection, it must be pulled back slightly and again inserted on the other side of the trunk to carry out another injection.
In the second technique, the spraying possibility given by the EUS 20 CPN needle allows the endosonographer to put the needle tip anterior to the basis of the origin of the celiac axis and to carry out only one injection (Figure 7).
Figure 7. EUS image of the needle at base of the celiac trunk.
The injection time is identical for both devices used. When the needle tip is in place, the inner sheet is removed and an aspiration test is performed to rule out vessel penetration before injection.
Then, 3-6 mL of a local analgesic, usually bupivacaine 0.25-0.75%, is injected first followed by 15-20 mL of a neurolytic agent (98%) dehydrate alcohol (Figure 8).
Figure 8. EUS image of lidocaine injection.
The alcohol injection produces an echogenic cloud obscuring the aorta and celiac axis (Figure 9).
Figure 9. EUS image of alcohol injection showing an echogenic cloud obscuring aorta and celiac axis.
In chronic pancreatitis patients, some Authors prefer to use steroids (10 mL or 80 mg (6 mL) triamcinolone) instead of alcohol.
However, in chronic pancreatitis, most results are obtained with alcohol and when traditional techniques were used.
The EUS CPN procedure usually lasts approximately 15 minutes and, during the procedure, arterial pressure has to be monitored because the alcohol injection may produce hypotension and it is necessary to infuse saline solution.
Results
Currently, there are few data about CPN under EUS guidance. However, the results are comparable to other conventional methods used to relieve pancreatic pain with neurolytic agent injections.
The safety and efficacy of EUS CPN has previously been demonstrated as relieving pancreatic pain in a cohort of 25 patients with pancreatic cancer followed for 12 weeks and 5 patients with other intra-abdominal malignant neoplasms [5]. These studies showed a significant decrease of pain at 2, 4, 8 and 12 weeks after EUS CPN.
About 80% of the patients also benefited in a long-term observation (a mean follow up-of 10 weeks) [5]. Other Authors described 58 patients treated in order to palliate pain due to non-operable pancreatic cancer. A short-term decrease of pain was seen in 78% of the patients but the control of pain decreased in 30% at 12 weeks.
Of particular interest is the evidence, reported by Gunaratnam et al. in the widest population reported to have been treated using this modality, that, if the treatment is associated with chemoradiation or chemotherapy, the decrease in the pain score was significantly higher as compared to patients who did not undergo any additional therapy [6] (Figure 10).
Figure 10. Decrease of the pain score according to different treatments. (N Gunaratnam et al. [6], modified).
Up to now, the real role of EUS CPN in the treatment of pain related to chronic pancreatitis is not so clear, lacking enough comparative data comparing EUS CPN and other modalities of treating such multifactorial pain.
Only two studies [7, 8] have tested EUS CPN in this setting; in 90 patients steroids were injected during EUS CPN and a beneficial effect was observed after 7 days in only 55% [7]. Furthermore, in the follow-up of these patients, only 25% still showed a significant decrease in the pain score after 12 weeks [7].
Many authors believe that the difference in results between patients with pancreatic cancer or chronic pancreatitis probably depends on the origin of the pain which can be considered only due to the nervous growing of the tumor in pancreatic cancer and which is multifactorial, with a great psychological impact, in chronic pancreatitis.
Another study described 22 cases of CPN (10 EUS CPN and 12 CT CPN) which showed the benefit in 40% at 8 weeks under EUS guidance (30% at 24 weeks) and in 25% under CT guidance. But the Authors concluded that the number of patients was too limited [8].
There are only a few complications related to the procedure and they are described as only transitory [3]. Orthostatic hypotension or a transient diarrhea may frequently be described.
The infusion of liquid can contrast the hypotension, while the diarrhea is generally auto-limiting and does not exceed 24 hours. Only a few cases of chronic diarrhea have been described; other complications are: peri-pancreatic abscess, reversible paraparesis and a pseudo alcohol-induced aneurysm [9, 10, 11]. To prevent the abscess, it is important to carry out antibiotic prophylaxis.
Conclusions
Celiac plexus neurolysis during EUS appears to be a safe technique, without complications. It seems to control neoplastic pancreatic pain in a short time in about 90% of cases and in a long time in about 30%.
In the management of chronic pancreatitis pain, the role of EUS CPN is not so clear and only 50% of patients have a good reduction of pain within a short period of time. However, only 10% seem to show a benefit at 24 weeks. EUS CPN results seem to be comparable to the results obtained with the other procedures, although the numbers are still too low. An important advantage is that EUS CPN may be performed during bioptic staging of pancreatic cancer [12].
It is also not very clear if there is the possibility of performing more than one procedure of neurolysis.
In summary, EUS CPN is safe and effective for the palliation of patients with pain caused by unresectable pancreatic cancer. Chemotherapy with and without radiotherapy also significantly decreased pain scores.
In addition, the proximity of the posterior lesser curve of the stomach to the celiac plexus, the use of continuous real time visualization of the target area and the availability of the Doppler to assess the vasculature all facilitate accurate needle placement.
CPN under EUS guidance requires further investigation in order to identify the advantages of this approach over conventional percutaneous techniques. The ability to perform the procedure in conjunction with tumor staging and FNA may streamline the care of these patients. The use of EUS CPN earlier in the course of pancreatic cancer to alleviate pain should be encouraged.
References
Levy MJ, Wiersema MJ. EUS-guided celiac plexus neurolysis and celiac plexus block. Gastrointest Endosc 2003; 57:923-9. [More details]
Kappis M. Sensibilitat und lokale Anasthesie im chirurgischen Gebiet der Bauchhohle mit besonderer Berucksichtigung der splanchnicus-Aasthesie. Beitrage zur klinischen Chirurgie 1919; 115:161-75. [More details]
Lee JM. CT-guided celiac plexus block for intractable abdominal pain. J Korean Med Sci 2000; 15:173-8. [More details]
Eisenberg E, Carr DB, Chalmers CT. Neurolytic celiac plexus block for treatment of cancer pain: a meta-analysis. Anesth Analg 1995; 80:290-5. [More details]
Wiersema MJ, Wiersema LM. Endosonography-guided celiac plexus neurolysis. Gastrointest Endosc1996; 44:656-62. [More details]
Gunaratnam NT, Sarma AV, Norton ID, Wiersema MJ. A prospective study of EUS-guided celiac plexus neurolysis for pancreatic cancer pain. Gastrointest Endosc 2001; 54:316-24. [More details]
Gress F, Schmitt C, Sherman S, Ciaccia D, Ikenberry S, Lehman G. Endoscopic ultrasound-guided celiac plexus block for managing abdominal pain associated with chronic pancreatitis: a prospective single center experience. Am J Gastroenterol 2001; 96:409-16. [More details]
Gress F, Schmitt C, Sherman S, Ikenberry S, Lehman G. A prospective randomized comparison of endoscopic ultrasound- and computed tomography-guided celiac plexus block for managing chronic pancreatitis pain. Am J Gastroentero. 1999; 94:900-5. [More details]
Chan VW. Chronic diarrhea: an uncommon side effect of celiac plexus block. Anesth Analg 1996; 82:205-7. [More details]
Navarro-Martinez J, Montes A, Comps O, Sitges-Serra A. Retroperitoneal abscess after neurolytic celiac plexus block from the anterior approach. Reg Anesth Pain Med 2003; 28:528-30. [More details]
Kumar A, Tripathi SS, Dhar D, Bhattacharya A. A case of reversible paraparesis following celiac plexus block. Reg Anesth Pain Med 2001; 26:75-8. [More details]
Varadarajulu S, Wallace MB. Applications of endoscopic ultrasonography in pancreatic cancer. Cancer Control 2004; 11:15-22. [More details]
Article in PDF format
--------------------------------------------------------------------------------
Keywords Celiac Plexus /innervation; Endosonography; Pain; Pancreatic Neoplasms
Abbreviations CPN: celiac plexus neurolysis
CorrespondencePaolo Giorgio ArcidiaconoDivision of Gastroenterology and Gastrointestinal EndoscopyUniversity Vita-Salute San RaffaeleIRCCS San Raffaele HospitalVia Olgettina, 6020132 MilanoItalyPhone: +39-02.2643.2145Fax: +39-02.215.2559E-mail address: arcidiacono.paologiorgio@hsr.it
JOP Home page
- http://www.joplink.net/prev/200407/26.html
1 comment:
Informative post. Thank you for sharing that.
Flush door Manufacturer in India, Veneer Manufacturer in India
Post a Comment